Avexitide for PBH
Stage
Phase 3 ReadyDisease
Post-Bariatric HypoglycemiaPrevalence
~180K in the US and <50% in EUMechanism of Action
GLP-1 AntagonistRegulatory Designations
- Orphan in US and EU
- Breakthrough Therapy in US
About Post-Bariatric Hypoglycemia (PBH)
PBH is a severe complication of bariatric and other gastrointestinal surgeries
Obesity currently affects over 40% of US adults and by 2030, a projected 50% of US adults will be obese and 25% severely obese. The use of bariatric surgery to address obesity and related comorbidities has increased by more than 50% over the past decade, with further increases expected due to the addition of bariatric surgery to the treatment algorithm for uncontrolled diabetes.
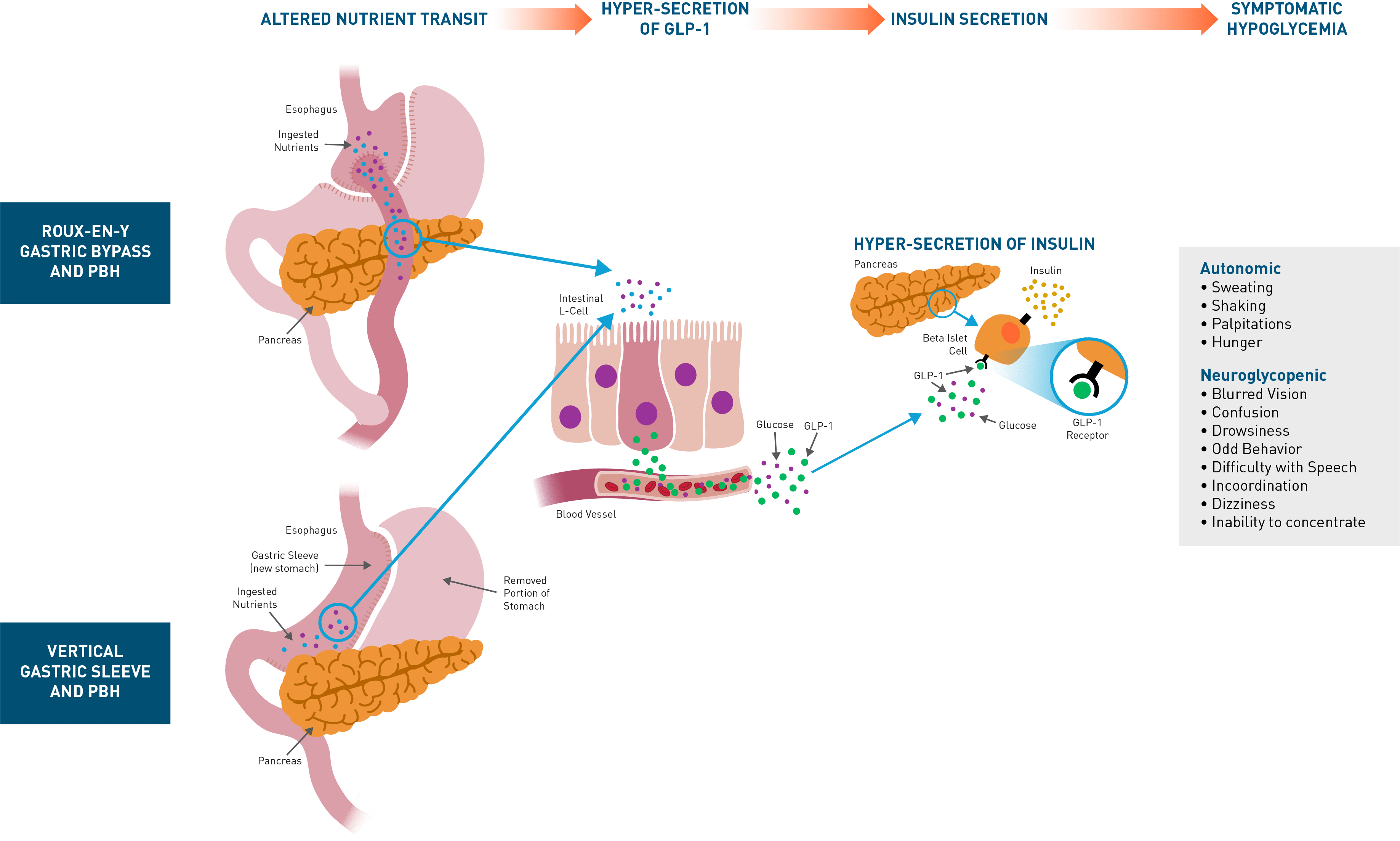
Post-bariatric hypoglycemia (PBH) is a rare but increasingly prevalent complication of bariatric surgery that affects approximately 10% of patients who undergo Roux-en-Y gastric bypass (RYGB) and 4% of those who undergo vertical sleeve gastrectomy (VSG). Patients who have other gastrointestinal surgeries, such as gastrectomy for gastric cancer, esophagectomy for esophageal cancer, or Nissen fundoplication for gastroesophageal reflux (GERD) can also be affected. This disorder leads to frequent episodes of symptomatic hypoglycemia, often resulting in glucose concentrations low enough to cause seizures, altered mental status, loss of consciousness, cognitive dysfunction, disability, and death. Quality of life can be severely diminished, and many patients cannot care for themselves or others, work, drive, or be left alone.
PBH can cause frequent episodes of severe hypoglycemia, putting patients at risk for seizures, motor vehicle accidents, coma, and death.
Avexitide is a well-characterized, first-in-class, GLP-1 receptor antagonist
Avexitide competes with endogenous GLP-1 for the GLP-1 receptor, counteracting the effects of excessive GLP-1 secretion. Avexitide has been shown in clinical studies to effectively prevent postprandial hyperinsulinemia and hypoglycemia and reduce neuroglycopenic symptoms in patients with PBH. Avexitide is a 31 amino acid peptide fragment of Exenatide, and has never been approved or commercialized for any indication. Avexitide has been dosed in 54 patients across four Phase 2 studies in patients suffering from Post-Bariatric Hypoglycemia. Avexitide has been granted Breakthrough Therapy Designation, as well as Orphan Drug Designation in the European Union by the EMA for the treatment of non-insulinoma Pancreatogenous Hypoglycemia Syndrome (NIPHS) and Orphan Drug Designation by the US FDA for the treatment of Hyperinsulinemic Hypoglycemia. Both of these broad designations include PBH. In clinical trials, avexitide has been generally well tolerated. Most commonly reported side effects have included transient, mild to moderate injection site pain, bruising, and/or reaction, nausea, dizziness, and headache.Avexitide: A 31 amino acid fragment of Exenatide*

*Exenatide is marketed as Byetta for type 2 diabetes and is well characterized.
Targeted blockade of GLP-1
PBH is characterized by frequent, symptomatic severe hypoglycemia. While mild cases can be managed with dietary modification, moderate-to-severe cases are refractory, and off-label use of medications (eg, octreotide, diazoxide, acarbose) is often poorly tolerated and/or incompletely efficacious. Consequently, a substantial unmet medical need remains for patients with PBH. The presence of inappropriately high insulin secretion after oral ingestion of nutrients has been well-established, with hyperinsulinemia occurring in response to oral but not intravenous glucose, due to exaggerated incretin effect. Plasma concentrations of glucagon-like peptide-1 (GLP-1), secreted by intestinal L-cells in response to oral nutrients are markedly elevated after meal intake in affected patients. GLP-1 is a gastrointestinal hormone secreted in response to meals that binds to GLP-1 receptors on the beta cells of the pancreas, enhancing the release of insulin and lowering blood glucose levels. In patients with PBH, exaggerated GLP-1 secretion results in dysregulated secretion of insulin and resultant symptomatic hypoglycemia.Avexitide: Designed to Normalize Insulin Secretion

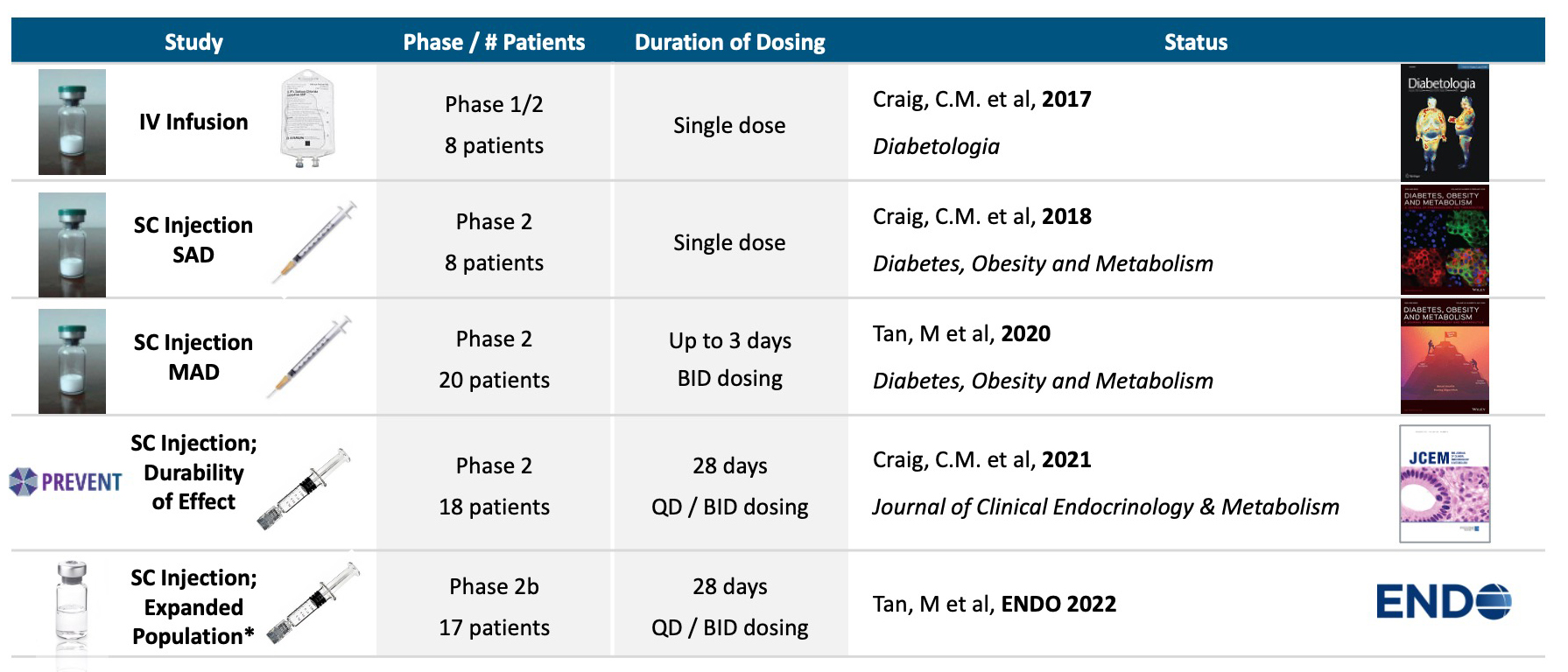
Avexitide clinical studies
Five proof of concept clinical studies in >70 patients with severe PBH have been completed with avexitide (below).Avexitide: Proof of Concept Demonstrated in Phase 2



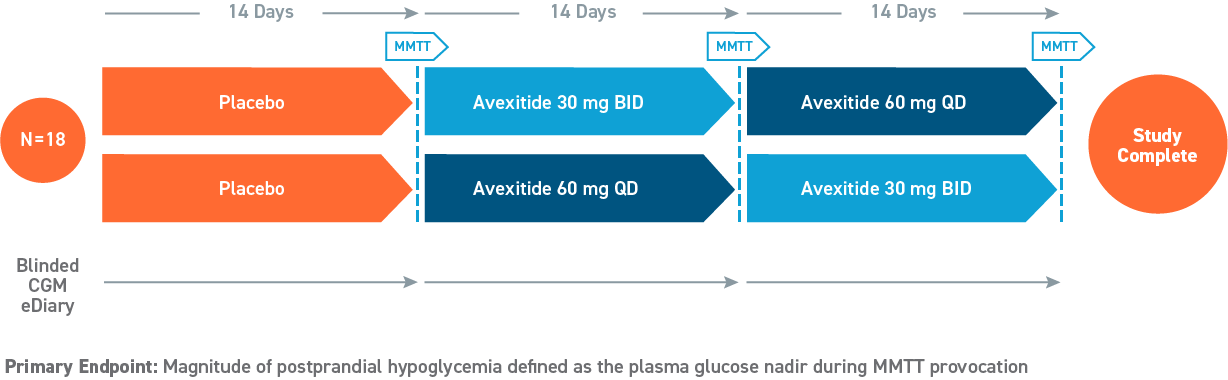
Phase 2, 28-day Study
(Swipe to interact)

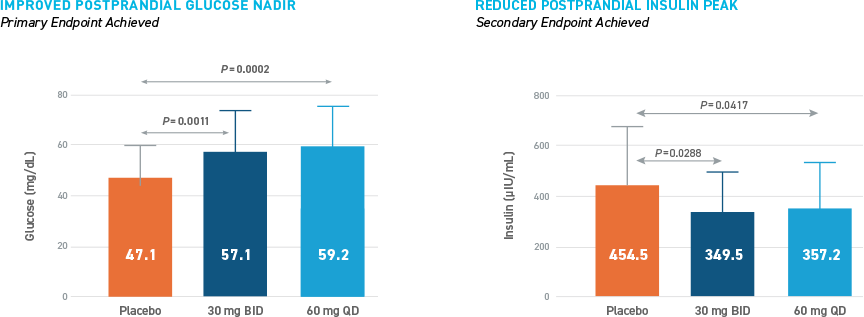

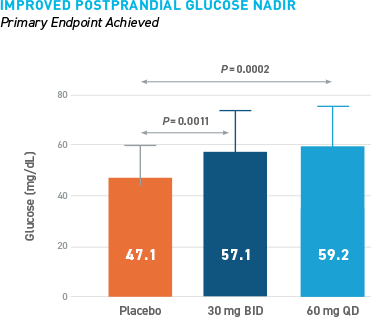

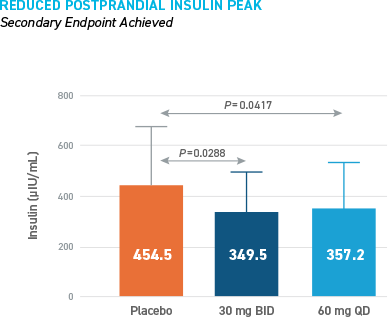

Reduced Hypoglycemia During Outpatient Assessment
CONSISTENT REDUCTIONS OBSERVED AS MEASURED BY SMBG, E-DIARY, CGM
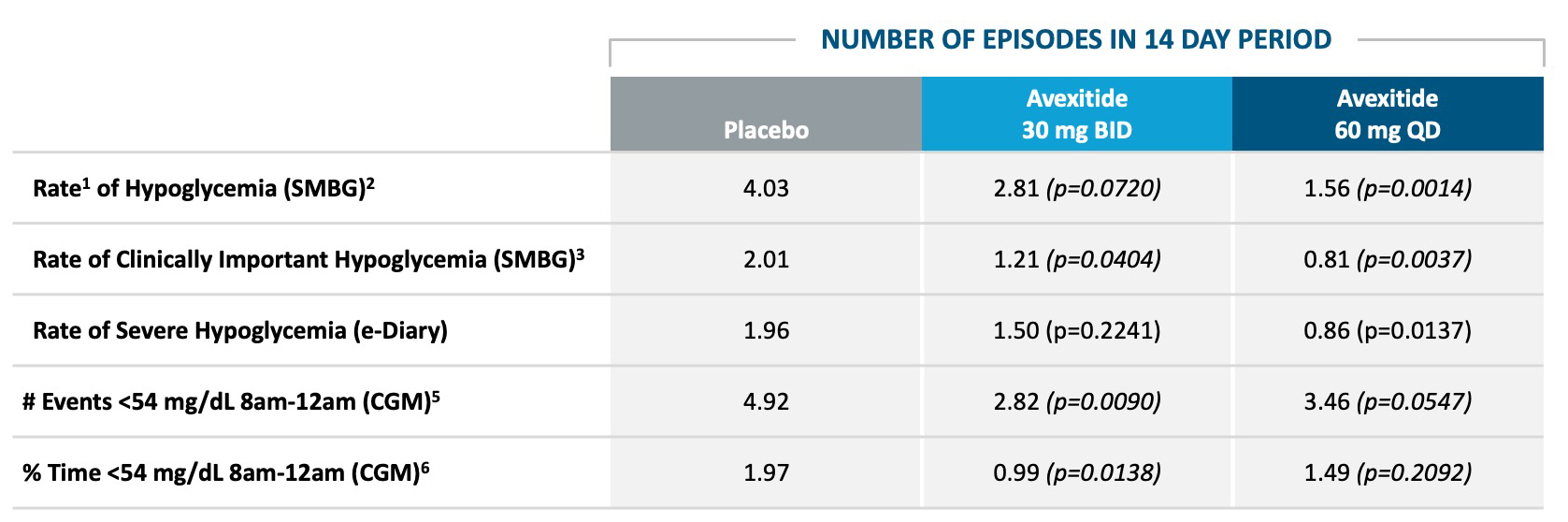

AVEXITIDE CLINICAL STUDIES IN PBH
- Tan, M. "Efficacy and Safety of Avexitidefor Treatment of Hypoglycemia after Gastrointestinal Surgery; Assessment of Novel Dosing Regimens in an Expanded Indication." ENDO 2022.
- Craig C et al. "PREVENT: A Randomized, Placebo-controlled Crossover Trial of Avexitide for Treatment of Postbariatric Hypoglycemia." J. Clin. Endocrin. Metab. 2021.
- Lee C et al. "28-Day dosing with avexitide Improves hyperinsulinemic hypoglycemia in patients with severe, refractory post-bariatric hypoglycemia: the PREVENT study." J Endocr Soc. 2019;3(Suppl 1):OR20-5.
- Craig CM et al. "Efficacy and pharmacokinetics of subcutaneous exendin (9-39) in patients with post-bariatric hypoglycaemia." Diabetes Obes Metab. 2018;20(2):352-361.
- Craig C et al. "Critical role for GLP-1 in symptmoatic post-bariatric hypoglycaemia." Diabetologia. 2017;60(3):531-540.
- Tan MJ et al. "Repeat subcutaneous dosing of exendin 9-39 reduces hyperinsulinemic hypoglycemia and neuroglycopenic symptoms in patients with post-bariatric hypoglycemia." Eiger BioPharmaceuticals. American Diabetes Association. June 9-13, 2017.
- Craig C, McLaughlin TL. "Subcutaneous exendin (9-39) effectively treats post-bariatric hypoglycemia." Presented at: American Diabetes Association, 76th Scientific Sessions; June 10-14, 2016; New Orleans, LA.
DISEASE OVERVIEW
- Patti ME, Goldfine AB. "The rollercoaster of post-bariatric hypoglycaemia." Lancet Diabetes Endocrinol. 2016;4(2):94-6.
- Angrisani L et al. "Bariatric Surgery Worldwide 2013." Obes Surg. 2015;25(10):1822-1832.
- Patti ME, Goldfine AB. "Hypoglycemia after gastric bypass: the dark side of GLP-1." Gastroenterology. 2014;146(3):605-8.
- Salehi M et al. "Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass." Gastroenterology. 2014;146(3):669-680.e2.
- Singh E, Vella A. "Hypoglycemia After Gastric Bypass Surgery." Diabetes Spectr. 2012;25(4):217-221.
